Softreg GmbH (RegCheck)
Compliance automation for medical device certification.
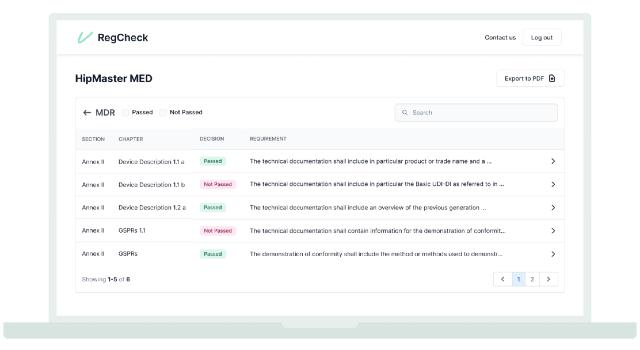
- The time and cost of certification of a medical device can be longer / higher than the design and development of the product itself because most of the certification applications are submitted incomplete, and therefore not approved by the certification authorities. We accelerate the time-to-market of medical devices by generating automatic gap analysis, predicting the audit outcomes of their technical files, avoiding the lenghty refusal rounds.
- We can do it because we have the regulatory, and software expertise in house with our 3 members of the core team.
- There are 35000 MedTech companies providing more than 500'000 medical devices (with a constant 6% market growth) to the European market and 10000 certification applications each year.
Only registered users have access to funding information.
Please log in or register to continue. Registration is free.
29.10.2025
Finalists revealed for three award competitions at Startup Nights (startupticker.ch)
No milestones
No Jobs
No videos and documents

Venture Kick
Venture Kick supports potential entrepreneurs with start capital up to CHF 150'000 as well as hands-on execution support and access to a nationwide network of investors and experts in the startup field. Multiple times per year, 8 selected projects get the opportunity to present their business ideas in front of a panel. The 5 most promising projects enter the support process. After 9 months at the latest, the startups incorporate and enter the market. Since 2007, Venture Kick has invested over CHF 58 million in over 1111 projects.

Softreg GmbH (RegCheck)
Compliance automation for medical device certification.
Website:
www.regcheck.ch
Headquarter:
Wohlen
Foundation Date:
March 2025
Technology:
- ICT
Sectors:
- Healthcare infrastructure
- Machine Learning / AI
- Medical devices
- Medtech
- SaaS
- Services for companies
- Software