AMF Medical SA
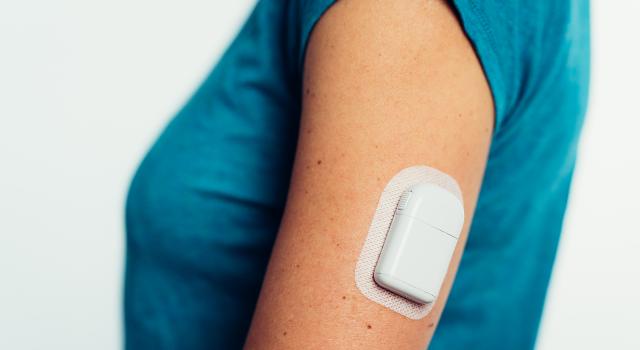
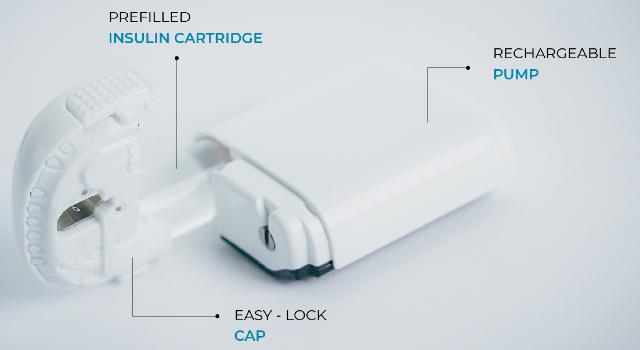
Sigi™ patch pump uniquely using prefilled insulin cartridges
Breakthrough Device designated (FDA) patch pump, to expand diabetes patient adoption and win, addressing unmet need to lower ‘therapy burden':
* Capturing share through unparalleled convenience and demonstrated, patient-preferred discretion and simplicity - uniquely - using commercially available pre-filled insulin cartridges; easier, smaller and lighter;
* Facilitating broader adoption through smart phone-controlled, secure, open-architecture interoperability with multiple 3rd-party Continuous Glucose Monitors and dosing algorithms, for Automated Insulin Delivery;
* Expanding clinically indicated population safely with independently validated best-in-class occlusion detection and high precision;
* At very significantly lower cost to manufacture and much reduced environmental impact.
Only registered users have access to funding information.
Please log in or register to continue. Registration is free.
16.12.2022
AMF Medical SA sold in CHF 200 million exit deal (startupticker.ch)
03.12.2021
AMF Medical and Sensars receive FDA Breakthrough Device Designation (startupticker.ch)
08.09.2021
CHF 500’000 for AMF Medical Insulin Pump (startupticker.ch)
No milestones
No Jobs
No videos and documents
No Awards

AMF Medical SA
Sigi™ patch pump uniquely using prefilled insulin cartridges
Website:
sigipump.com/
Headquarter:
Ecublens
Foundation Date:
July 2014
Technology:
- Medtech
Sectors:
- Micro technologies
- Medical devices
- Medtech
- Wearable technologies